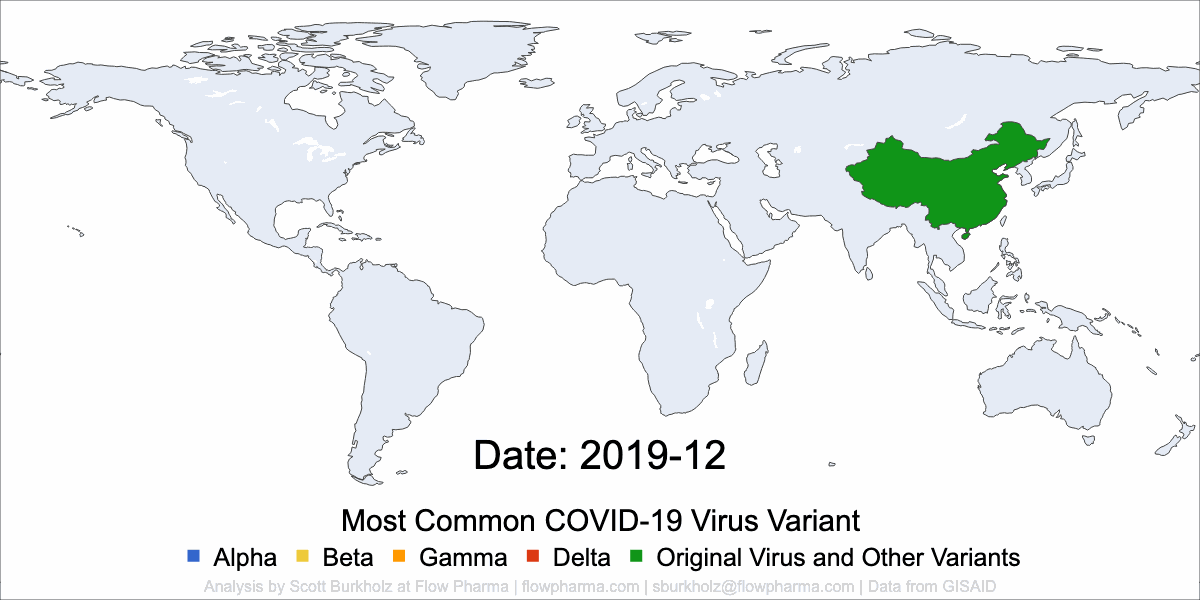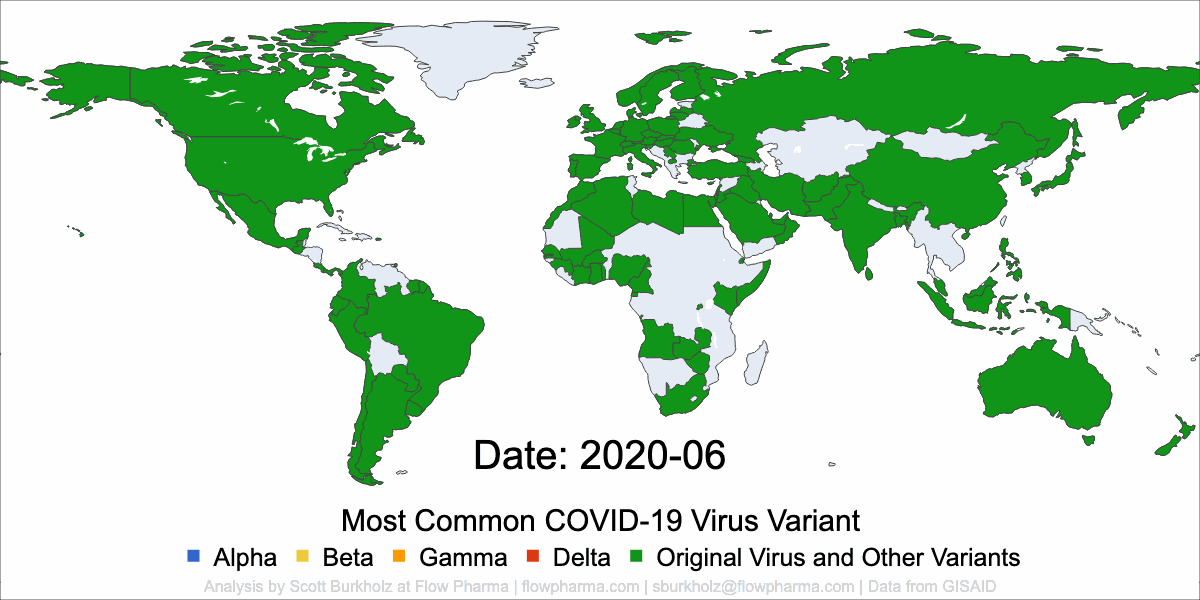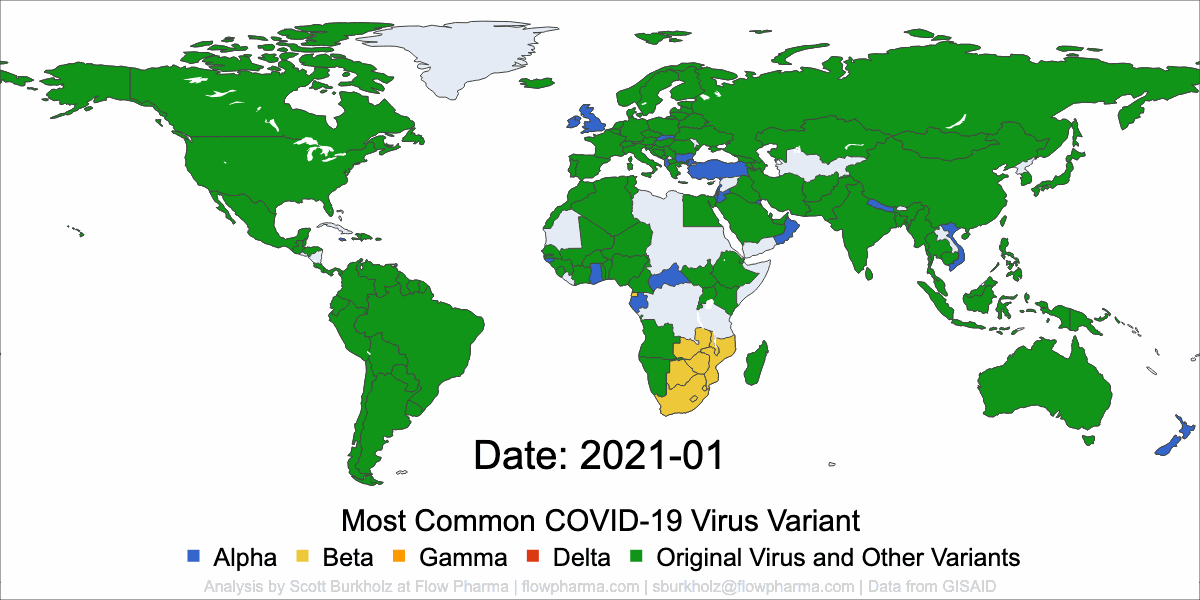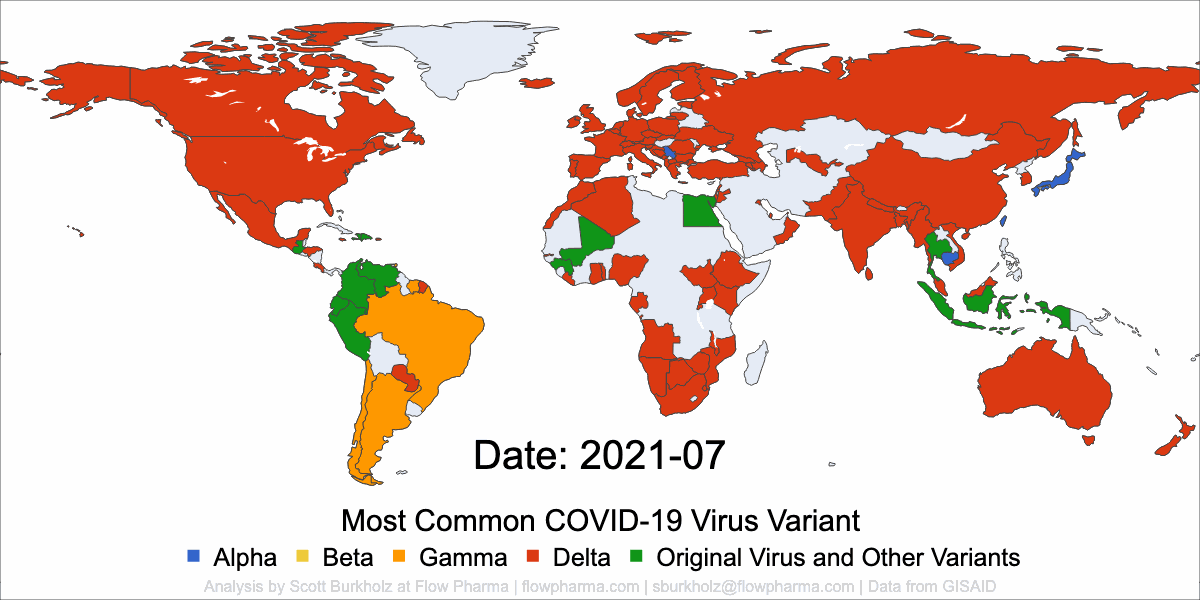
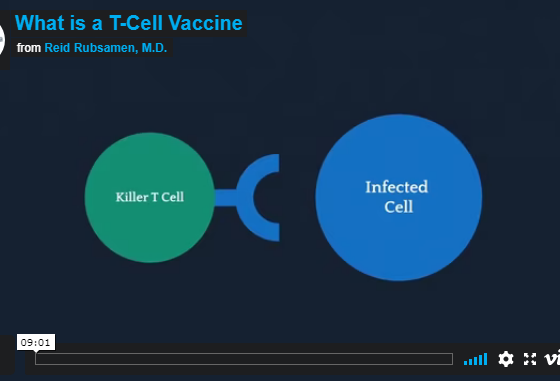
Flow Pharma is dedicated to immunotherapy innovation.
Our exclusive technology and manufacturing expertise are poised to transform the treatment and prevention of cancer and viral disease.
News & Press