Our Mission
Flow Pharma is dedicated to immunotherapy innovation.
Our exclusive technology and manufacturing expertise are poised to transform the treatment and prevention of cancer and viral disease.
Product Portfolio
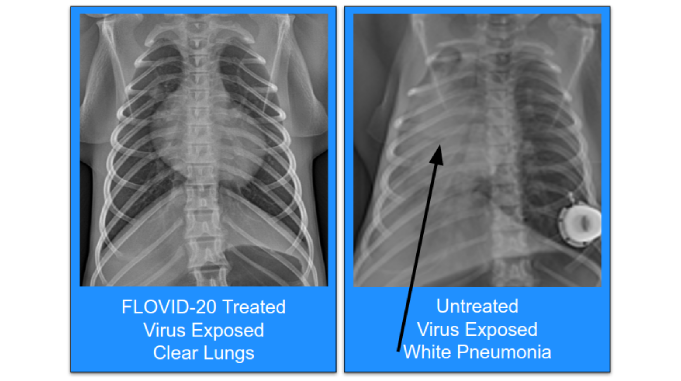
FLOVID-20
COVID-19, FLOVID-20
An inhaled or injected treatment to prevent COVID-19 disease in both virus-exposed and healthy patient populations.
FlowVax Ebola/Marburg
Ebola, Marburg
Marburg is a weaponized Ebola-like virus. By stimulating T-cells to target these viruses, long-lasting infection prevention is attainable.