Targeted T-cell Immunotherapy
A treatment for new COVID-19 patients. Administered by inhaler or injection.
Designed to prevent severe COVID-19 disease and cure COVID ‘long-haulers’.
Vaccination by Inhalation or Injection. FLOVID-20 can use inhalation or injection to deliver a preventative and therapeutic immuno-stimulatory biomedicine targeting nucleocapsid epitopes on SARS-CoV-2.
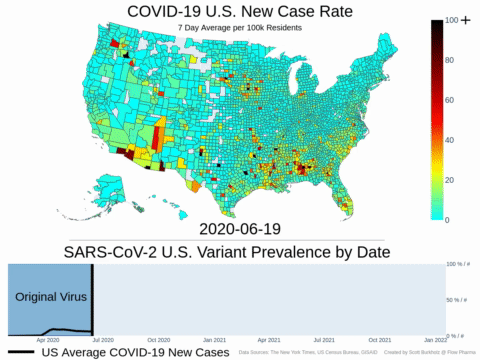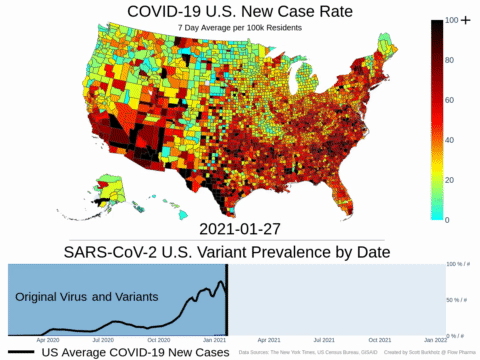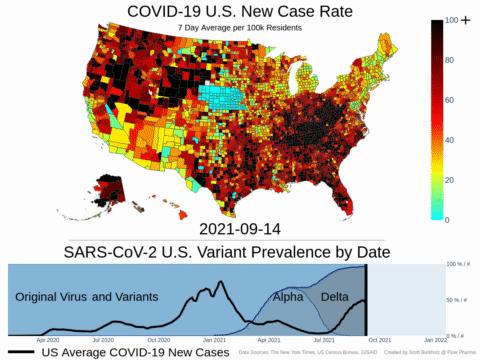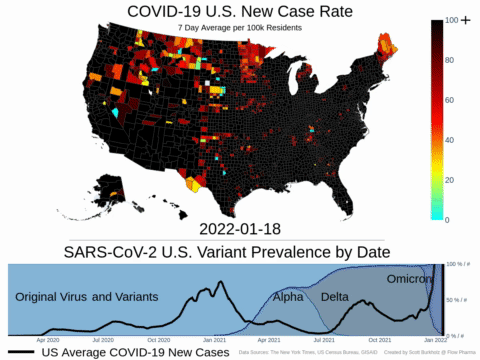
Mutations of COVID-19 are frequent and unpredictable—FLOVID-20 is targeting the virus at places predicted by Flow Pharma scientists to be least likely to mutate. So far, none of the known COVID-19 virus variants have mutations in those locations.
Overview
- Under Development, FDA Approval Required.
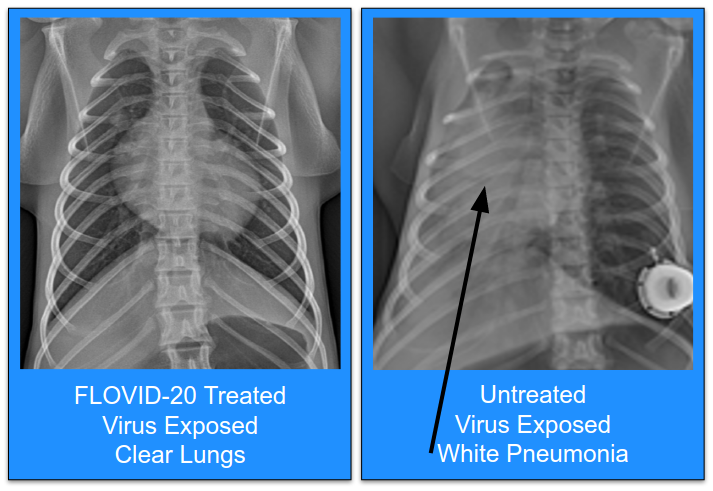
- In Spring 2020, FLOVID-20 non-human primate studies were performed at the United States National Laboratory, University of Texas Medical Branch, Galveston
- Treated primates resisted infection. Control primates developed COVID pneumonia.
Current Status
-
PRE-CLINICAL FEASIBILITY
-
MANUFACTURING FOR CLINICAL TESTING
-
PHASE I/II CLINICAL TESTING
How Does Our Therapy Work?
What is COVID-19 and SARS-CoV-2?
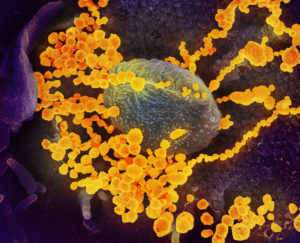
COVID-19 is the disease caused by Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
This scanning electron microscope image shows SARS-CoV-2 (round gold objects) emerging from the surface of cells cultured in the lab. The virus shown was isolated from a patient in the U.S. Image captured and colorized at NIAID’s Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
