Flow Pharma is creating FlowVax HPV, an immunotherapy designed to work synergistically with existing treatment options to address HPV-induced cancers.
HPV causes a particularly deadly form of skin cancer in organ transplant patients. Current HPV vaccines do not cover the oncogenic strains of the virus responsible for these tumors. Flow Pharma is designing an HPV vaccine specifically addressing this unmet need.
Overview
- FlowVax HPV is under development. FDA Approval is Required.
- HPV (human papillomavirus) is the cause of virtually all cervical cancers and most oral-pharyngeal squamous cell cancers
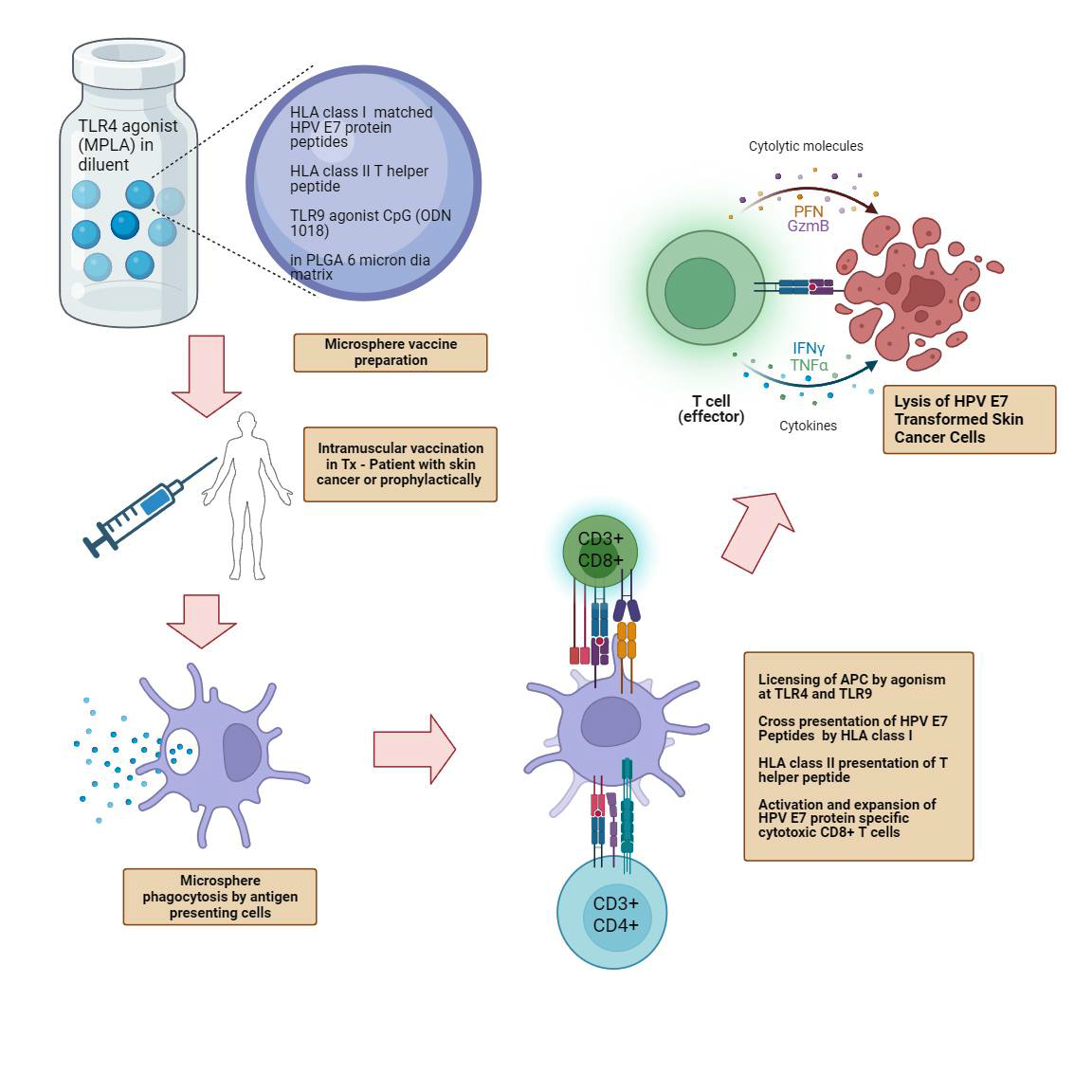
- Our T-cell immunotherapy for cervical and head & neck cancer targets HPV E6 and E7 Oncoproteins.
- FlowVax HPV is delivered by intranodal injection.
Status
-
Pre-Clinical Feasibility
-
Phase I
What is HPV-Induced Cancer?
Human papillomavirus (HPV) is a common virus, spread from person to person, that is best known for causing warts on various skin and mucosal surfaces. While sometimes unsightly and always unwanted, most HPV infections cause no symptoms and resolve spontaneously within two years. Occasionally, HPV infection persists and undergoes a precancerous transformation. These precancerous lesions, depending on the site affected (and other factors), are at risk of further progression to cancer. HPV induced cancers of the uterine cervix, vulva, vagina, penis, anus, mouth, and throat are common. Nearly all cervical cancer is caused by HPV. Even when an HPV infection visibly resolves, the changes HPV causes in the DNA of infected cells persist, contributing to the development of HPV-induced squamous cell carcinoma, a dangerous but common form of cancer.
The route from HPV infection to cancer is complex. Of the more than 100 strains of HPV, types 16 and 18 are the two strains most responsible for human cancer. Papillomaviruses gain access to keratinocyte stem cells through small wounds, known as microtraumas, in the skin or mucosal surface, frequently transmitted during sexual activity, leading to cervical, penile and oral cancers.
While many factors are involved in cancer evolving from an HPV infection, two key viral onco(cancer)proteins play a central role, E6 and E7. They arise when there is integration of HPV DNA into infected human host cells, and there is subsequent E7 and E6 viral protein expression even after the HPV infection resolves clinically.
E6 and E7 work synergistically to drive cancer development. E6 blocks a key tumor suppressor protein, p53, reducing the cell’s ability to respond to DNA damage. Overall, the E6 protein serves to impede normal protein activity in such a way as to allow a cell to grow and multiply at an increased rate, a characteristic of cancer.
The expression of E6 is strictly required for maintenance of a malignant phenotype in HPV-induced cancers. E7 disables a key braking mechanism on cellular division, allowing for dysregulation of cellular multiplication, and a high proliferation rate to occur.
Since E6 and E7 are foreign proteins and are present on the surface of all HPV-induced cancers, they represent outstanding targets for directed cellular immunotherapy. For various reasons, host T-cell responses to E7 and E6 in HPV-induced cancers are frequently muted. FlowVax HPV is designed to stimulate a brisk natural immune response, overcoming T-cell suppression, creating an expanded class of precision-targeting killer T-cells that selectively induce death of E6/E7 bearing cancer cells. First generation immunotherapies have shown clinical efficacy via HPV specific T-cell stimulation to shrink tumors, but widespread efficacy has not been observed.
The scientists at Flow Pharma are optimistic that there will be a significant net benefit when FloVax HPV is included as part of a global treatment strategy for individual patients with HPV induced cancer.
